|

|
The
views expressed
on this page are soley those of the author and do not
necessarily
represent the views of County News Online
|
 |
The Daily Signal
Federal Agency
Faces Fallout From Controversial Study That Put Babies at Risk
Sharyl Attkisson
December 04, 2014
A federal agency has taken controversial new steps to marginalize its
own ethics body, according to new information revealed by the watchdog
group Public Citizen. That after the federal ethics body criticized a
government study that resulted in the deaths of some premature babies.
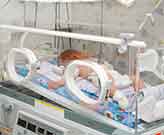
The government-backed study in question was called “SUPPORT.” It was
conducted from 2006 to 2009 on 1,316 extremely premature infants at 23
academic institutions under the National Institutes of Health, which is
part of the Department of Health and Human Services.
Controversy surfaced in March 2013 when the government’s own ethics
body, the Office of Human Research Protections, dropped a bombshell.
The ethics office found the federal study’s consent forms violated
government rules designed to protect human research subjects. According
to OHRP, the consent forms “failed to describe the reasonably
foreseeable risks of blindness, neurological damage and death” to
babies in the study.
More than a year and a half later, the federal government has yet to
take enforcement action or make changes to address the shortfalls.
Instead, critics say it has pressured and marginalized the ethics
office that made the critical findings.
‘Conflict of interest’
Fallout over the study has impacted the federal government’s current
review of the longstanding rules that govern research on human test
subjects. Watchdogs say the effort has been co-opted by the powerful
research community, which has used the opportunity to push for a
loosening—rather than strengthening—of human research consent rules.
Now, as the rules are being revised, internal emails obtained by Public
Citizen indicate federal officials have transferred major
responsibilities from the ethics office and assigned them to the
National Institutes of Health, the very agency that conducted the
questioned study.
Public Citizen’s Dr. Michael Carome calls that an “obvious, direct
conflict of interest.” In a Nov. 20 letter to Health and Human Services
Secretary Sylvia Mathews Burwell, he asks HHS to reverse the move.
“This shift of authority from the regulator to the regulated is
unacceptable,” writes Carome, who once served as an ethics expert in
OHRP.
HHS has not respond to Public Citizen’s letter and did not answer our
request for comment.
'I didn't have a clue': Sharrissa Cook, Dreshan's mom, with her father.
(Photo: Angela Bradbery/Public Notice)
‘I didn’t have a clue': Sharrissa Cook, Dreshan’s mom, with her father.
(Photo: Angela Bradbery/Public Citizen)
Parental outrage
A number of parents have come forward to say they were misled when they
enrolled their extremely ill newborns into the SUPPORT study, which
stands for “Surfactant, Positive Airway Pressure, and Pulse Oximetry
Randomized Trial.”
Parents say they were told it was just an effort to help their baby or
gather data such as height and weight. Among other risks, the consent
forms failed to disclose that oxygen monitors used on the study babies
were intentionally rigged to give false readings...
Read the rest of the article with links and photos and The Daily Signal
|
|
|
|

